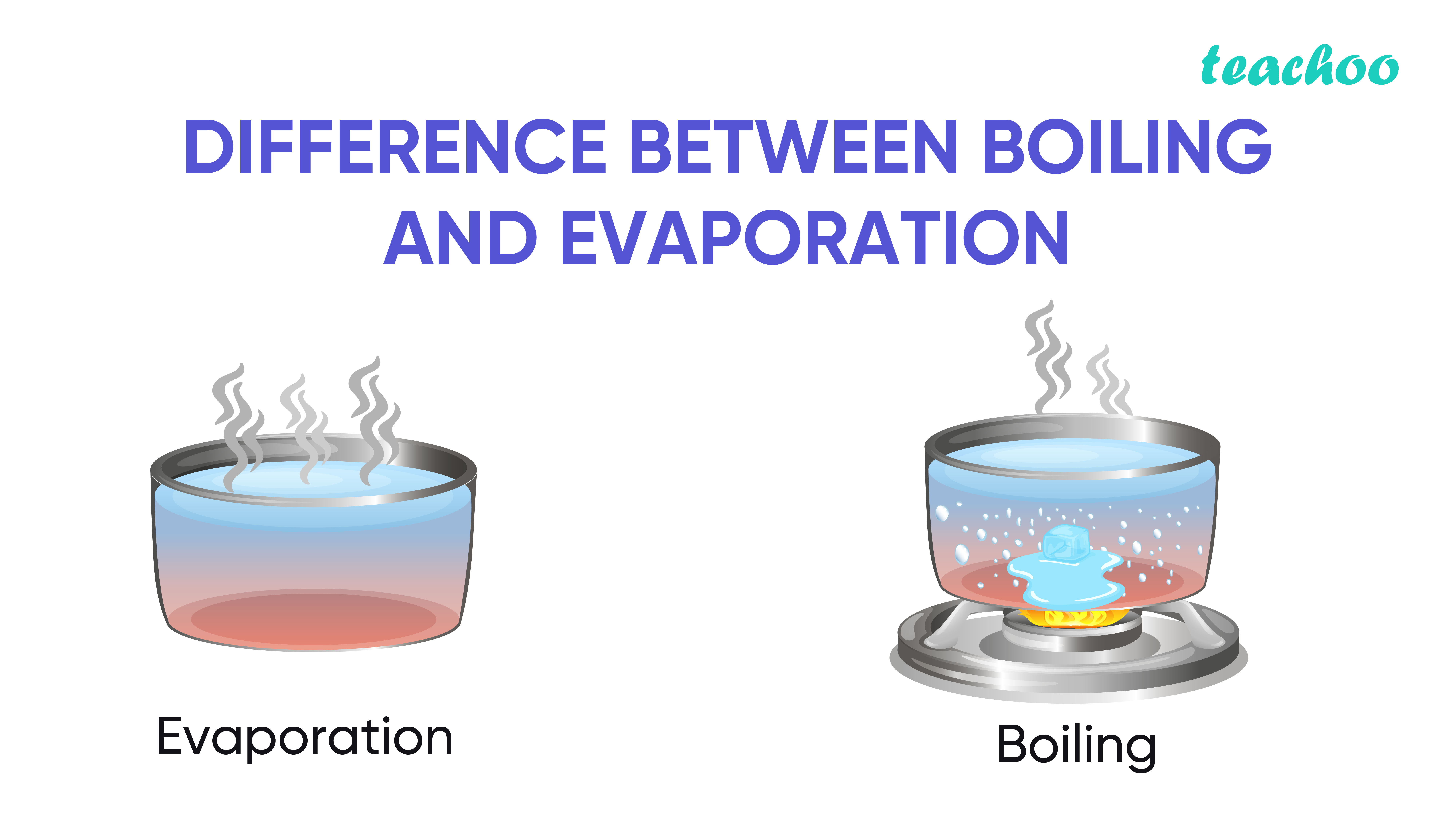
Evaporation Heat Water . evaporation is the process that changes liquid water to gaseous water (water vapor). specific heat capacity and heat of vaporization of water. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. evaporation happens when a liquid substance becomes a gas. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. The heat required to evaporate 10 kg. Water moves from the earth’s. When water is heated, it e vaporates.
from www.teachoo.com
evaporation is the process that changes liquid water to gaseous water (water vapor). The heat required to evaporate 10 kg. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. evaporation happens when a liquid substance becomes a gas. specific heat capacity and heat of vaporization of water. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. When water is heated, it e vaporates. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. Water moves from the earth’s.
What is the Difference between Evaporation and Boiling? Class 9
Evaporation Heat Water evaporation happens when a liquid substance becomes a gas. Water moves from the earth’s. When water is heated, it e vaporates. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. evaporation happens when a liquid substance becomes a gas. specific heat capacity and heat of vaporization of water. The heat required to evaporate 10 kg. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. evaporation is the process that changes liquid water to gaseous water (water vapor).
From www.openaccessgovernment.org
Lightinduced evaporation Study shows light can trigger water Evaporation Heat Water Water moves from the earth’s. specific heat capacity and heat of vaporization of water. evaporation happens when a liquid substance becomes a gas. The heat required to evaporate 10 kg. evaporation is the process that changes liquid water to gaseous water (water vapor). the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure. Evaporation Heat Water.
From www.bbc.co.uk
Evaporation BBC Bitesize Evaporation Heat Water Water moves from the earth’s. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. evaporation happens when a liquid substance becomes a gas. When water is heated,. Evaporation Heat Water.
From www.youtube.com
SingleEffect Evaporator Heat Transfer Area YouTube Evaporation Heat Water the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. evaporation is the process that changes liquid water to gaseous water (water vapor). the (latent) heat of vaporization. Evaporation Heat Water.
From www.youtube.com
Thermal Evaporation What is it and how does it work? YouTube Evaporation Heat Water the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. evaporation happens when a liquid substance becomes a gas. When water is heated, it e vaporates. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. specific heat. Evaporation Heat Water.
From www.processtechacademy.com
Proc Tech & Oper Acad Sensible & Latent Heat Evaporation Heat Water evaporation happens when a liquid substance becomes a gas. When water is heated, it e vaporates. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. evaporation is the process that changes liquid water to gaseous water (water vapor). The heat required to evaporate 10. Evaporation Heat Water.
From www.youtube.com
What is evaporation How salt is made Evaporation process & facts Evaporation Heat Water evaporation happens when a liquid substance becomes a gas. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. Water moves from the earth’s.. Evaporation Heat Water.
From www.researchgate.net
Schematic illustration of solardriven interfacial water evaporation Evaporation Heat Water as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. evaporation is the process that changes liquid water to gaseous water (water vapor). When water is heated, it e vaporates. evaporation happens when a liquid substance becomes a gas. specific heat capacity and heat of. Evaporation Heat Water.
From www.youtube.com
Evaporation and Condensation of Water Drop Simultaneous Heat and Mass Evaporation Heat Water specific heat capacity and heat of vaporization of water. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. The heat required to evaporate 10 kg. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount. Evaporation Heat Water.
From www.thoughtco.com
How Condensation and Evaporation Shape Our Weather Evaporation Heat Water evaporation happens when a liquid substance becomes a gas. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the (latent) heat of vaporization (∆h vap ) also. Evaporation Heat Water.
From www.pinterest.com
EvaporatorApplications Of Refrigeration Refrigeration and air Evaporation Heat Water When water is heated, it e vaporates. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. Water moves from the earth’s. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. The heat required to evaporate 10 kg. Web. Evaporation Heat Water.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Evaporation Heat Water evaporation is the process that changes liquid water to gaseous water (water vapor). evaporation happens when a liquid substance becomes a gas. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. specific heat capacity and heat of vaporization of water. The heat required. Evaporation Heat Water.
From www.oceanproperty.co.th
Evaporation, Condensation, And Dew Point, 56 OFF Evaporation Heat Water Water moves from the earth’s. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. The heat required to evaporate 10 kg. specific heat capacity and heat of vaporization of water. evaporation is the process that changes liquid water to gaseous water (water vapor). evaporation happens when a liquid. Evaporation Heat Water.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Evaporation Heat Water the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. When water is heated, it e vaporates. specific heat capacity and heat of vaporization of water. evaporation happens when a liquid substance becomes a gas. Water moves from the earth’s. the latent heat of. Evaporation Heat Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock Evaporation Heat Water evaporation happens when a liquid substance becomes a gas. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. The heat required to evaporate 10 kg. evaporation is the process that changes liquid water to gaseous water (water vapor). Water moves from the earth’s. Web. Evaporation Heat Water.
From electricalworkbook.com
What is Forced Circulation Evaporator? Working Principle, Diagram Evaporation Heat Water evaporation is the process that changes liquid water to gaseous water (water vapor). evaporation happens when a liquid substance becomes a gas. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the (latent) heat of vaporization (∆h vap ) also known as the enthalpy. Evaporation Heat Water.
From www.researchgate.net
Energy balance diagram of a solar water evaporation system based on Evaporation Heat Water the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. When water is heated, it e vaporates. Water moves from the earth’s. evaporation happens when a liquid substance becomes a gas. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and. Evaporation Heat Water.
From www.lcicorp.com
Selecting Evaporators for Process Applications Evaporation Heat Water The heat required to evaporate 10 kg. Water moves from the earth’s. as a result of the network of hydrogen bonding present between water molecules, a high input of energy is required to. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c. the (latent) heat of vaporization (∆h vap. Evaporation Heat Water.
From www.iqsdirectory.com
Wastewater Evaporator What Is It? How Does It Work? Types Evaporation Heat Water Water moves from the earth’s. evaporation is the process that changes liquid water to gaseous water (water vapor). the (latent) heat of vaporization (∆h vap ) also known as the enthalpy of vaporization or evaporation, is the amount of energy. the latent heat of evaporation for water is 2256 kj/kg at atmospheric pressure and 100 o c.. Evaporation Heat Water.